Piperacillin/tazobactam in modern clinical practice
Abstract: Piperacillin/tazobactam represents a combination of the ureidopenicillin and the β-lactamase inhibitor. The antibiotic is the drug of choice for the treatment of severe infections, primarily in surgical and intensive care units. The area of clinical use of piperacillin/tazobactam includes intra-abdominal infections, skin and soft tissues infections, late nosocomial pneumonias, including ones in the context of the covid-19 pandemic, and infections of other localizations.
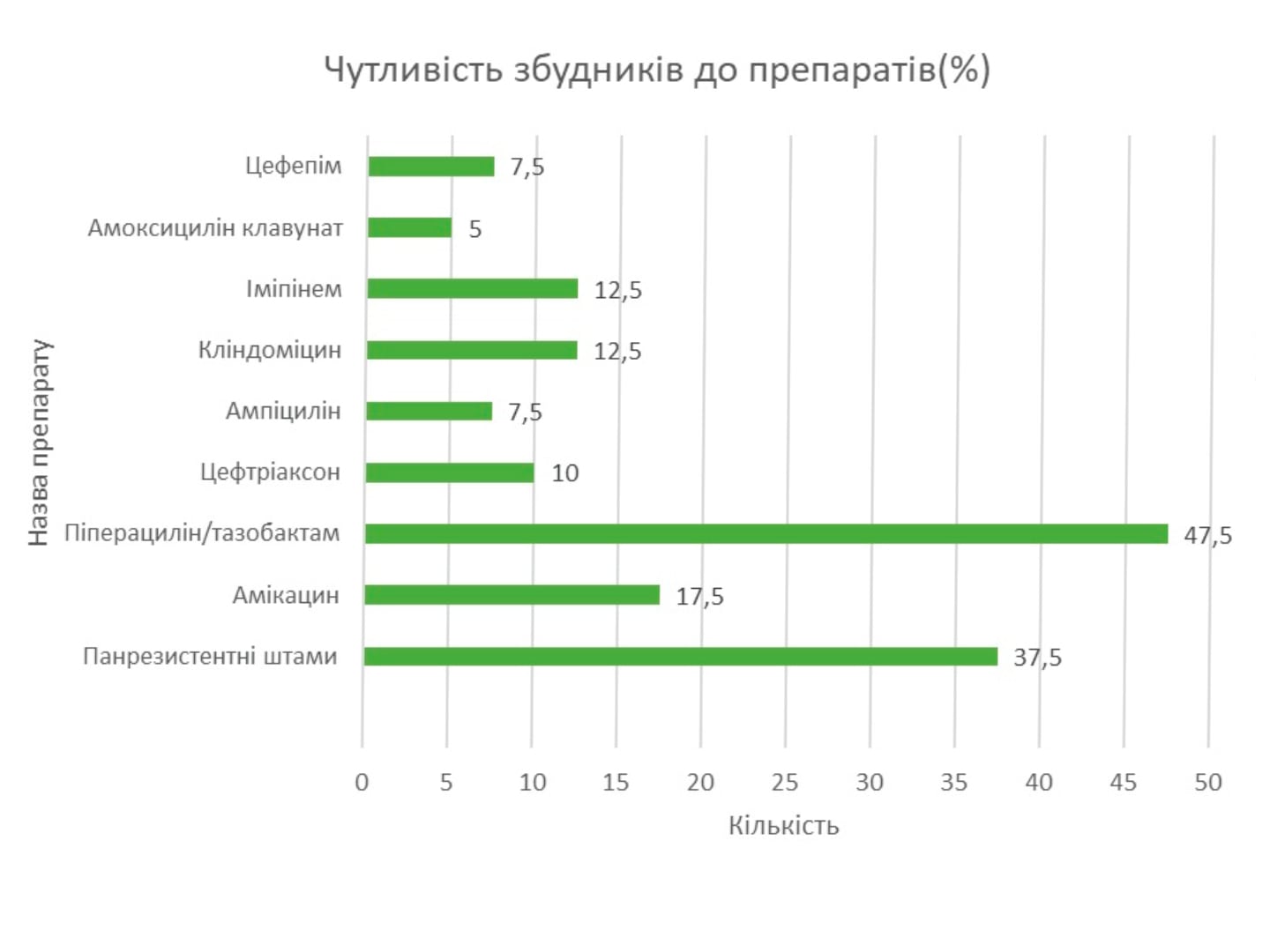
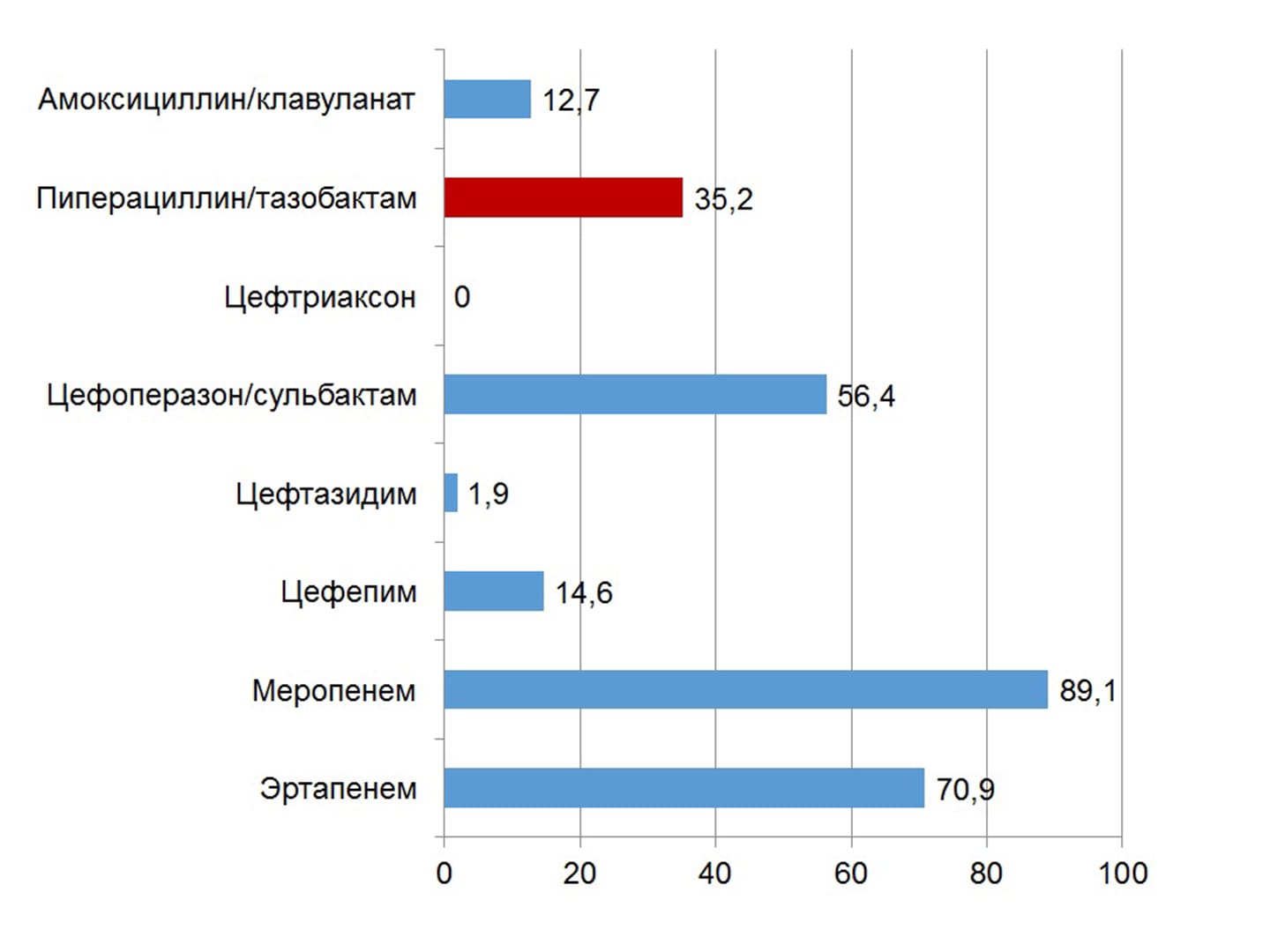
The effectiveness of the intervention increases with intravenous administration of the drug by the method of prolonged infusions. A high susceptibility of most causative pathogens of wound infection to piperacillin/tazobactam has been established in Ukraine (with the exception of Klebsiella spp. and non-fermenting bacteria). The drug can be used in patients with a history of allergic reactions to β-lactams; preliminary allergological testing is indicated only in cases of allergy to aminopenicillins and aminocephalosporins. The first domestic piperacillin/tazobactam under the name Refex is registered on the Ukrainian market.
Key words: piperacillin/tazobactam, prolonged infusions, susceptibility to antibiotics, nosocomial pneumonias, Refex.
Authors:
I.G. Bereznyakov, Kharkov Medical Academy of Postgraduate Education
Literature:
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050684s88s89s90_050750s37s38s39lbl.pdf. Посещение 07.12.2021 г.
- Craig W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect.Dis.Clin. North Am. 2003; 17:479–501.
- Drusano G.L. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat.Rev.Microbiol. 2004; 2:289–300.
- McKinnon P.S., Paladino J.A., Schentag J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T > MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J.Antimicrob. Agents 2008; 31:345–351.
- Abdul-Aziz M.H., Lipman J., Mouton J.W., et al. Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: optimizing efficacy and reducing resistance development. Semin.Respir. Crit. Care Med. 2015;36(1):136–153.
- Roberts J.A., Abdul-Aziz M.H., Lipman J., et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect. Dis. 2014;14 (6):498–509.
- Imani S., Buscher H., Day R., et al. An evaluation of risk factors to predict target concentration non-attainment in critically ill patients prior to empiric β-lactam therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2018; 37 (11): 2171–2175.
- Alobaid A.S., Brinkmann A., Frey O.R., et al. What is the effect of obesity on piperacillin and meropenem trough concentrations in critically ill patients? J.Antimicrob.Chemother. 2016;71(3):696–702.
- Evans L., Rhodes A., Alhazzani W., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intens. Care Med. https://doi.org/10.1007/s00134-021-06506-y.
- Fawaz S., Barton S., Nabhani-Gebara S. Comparing clinical outcomes of piperacillin-tazobactam administration and dosage strategies in critically ill adult patients: a systematic review and metaanalysis. BMC Infect. Dis. 2020; 20: 430.
- Yang H., Cui X., Ma Z., et al. Clinical outcomes with alternative dosing strategies for piperacillin/tazobactam: a systematic review and meta-analysis. PLoS One. 2015; 19 (2): 274–89.
- Abdulla A., Ewoldt T.M.J., Purmer I.M., et al. A narrative review of predictors for β-lactam antibiotic exposure during empirical treatment in critically ill patient. Expert Opin. Drug Metabol. Toxicol. 2021; 17 (4): 359-368.
- Протоколнаданнямедичноїдопомогихворимнанегоспітальнутанозокоміальну (госпітальну) пневмоніюудорослихосіб: етіологія, патогенез, класифікація, діагностика, антибактеріальнатерапія. Затверджений наказом МОЗ Українивід 19 березня 2007 р. № 128. Доступно за адресою: http://www.ifp.kiev.ua/doc/staff/MOZ-128-19032007.pdf.
- Kalil A.C., Metersky M.L., Klompas M., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016; 63 (5): e61–111.
- Torres A., Niederman M.S., Chastre J., et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia.Eur.Respir. J. 2017; 50: 1700582.
- Pneumonia (hospital-acquired): antimicrobial prescribing. NICEguideline [NG139]. Доступно по адресу: https://www.nice.org.uk/guidance/ng139
- Протокол «надання медичної допомоги для лікування коронавірусної хвороби (covid-19)». Оновлений 11.11.2021 р. Доступно за адресою:https://moz.gov.ua/article/ministry–mandates/nakaz–moz–ukraini–vid-11112021–2495-pro–vnesennja–zmin–do–protokolu–nadannja–medichnoi–dopomogi–dlja–likuvannja—koronavirusnoi–hvorobi–covid-19
- SfeirM.M., AskinG., ChristosP. Beta-lactam/beta-lactamase inhibitors versus carbapenem for bloodstream infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2018;52(5):554-570.
- Harris P.N.A., Tambyah P.A., Lye D.C., et al. Effect of piperacillin-tazobactam vs meropenem on 30-daymortality for patients with E coli or Klebsiellapneumoniaebloodstream infection and ceftriaxone resistance. A randomized clinical trial. JAMA 2018; 320: 984–94.
- Andersen M.G., Thorsted A., Storgaard M., et al. Population pharmacokinetics of piperacillin in sepsis patients: should alternative dosingstrategies be considered? Antimicrob. Agents Chemother. 2018; 62: e02306–17.
- Pitout J.D., Le P., Church D.L., et al. Antimicrobial susceptibility of well-characterisedmultiresistant CTX-M-producing Escherichia coli: failure of automated systems to detect resistance to piperacillin/tazobactam. Int. J. Antimicrob. Agents 2008; 32: 333–8.
- Henderson A., Paterson D.L., Chatfield M.D., et al. Association between minimum inhibitory concentration, β-lactamase genes and mortality for patientstreated with piperacillin/tazobactam or meropenem from the MERINO study.Clin. Infect. Dis. 2020; doi: 10.1093/cid/ciaa1479
- Rodríguez-Baño J., Gutiérrez-Gutiérrez B., Pascual A. CON: Carbapenems are NOT necessary for all infections caused by ceftriaxone-resistant Enterobacterales. JAC Antimicrob. Resist. 2021. doi:10.1093/jacamr/dlaa112
- Takimoto K., Wang Q., Suzuki D., et al. Clinical efficacy of piperacillin/tazobactam in the treatment of complicated skin and soft tissue infections. Expert Opinion Pharmacother., 2017. doi:10.1080/14656566.2017.1341491
- Stevens D.L., Bisno A.L., Chambers H.F., et al. Practice guidelines for the diagnosis andmanagement of skin and soft tissue infections: 2014 update by the Infectious Diseases Society ofAmerica. Clin. Infect. Dis. 2014;59:e10–52.
- Lipsky B.A., Berendt A.R., Cornia P.B., et al. 2012 Infectious Diseases Society of Americaclinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect .Dis. 2012;54:e132–73.
- Diabetic foot problems: prevention and management. NICE guideline. Last updated 11 October 2019. Доступно по адресу: www.nice.org.uk/guidance/ng19
- Березняков І.Г. Стан антибіотикорезистентності в Україні: результати дослідження АУРА. Здоров’я України ХХІ вік. 2020; тематичний номер (4): 31-34.; тематичний номер (5): 21-23.
- Macy E., Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J. Allergy Clin. Immunol. 2014; 133: 790-6.
- Zagursky R.J., Pichichero M.E. Cross-reactivity in β-lactam allergy J. Allergy Clin. Immunol. Pract. 2018; 6: 72-81.e1.
- Березняков И.Г. Аллергические реакции на антибиотики. Харьков: ГО УАДВА, 2021. – 58 с.