Infusion GIK solutions
Bogomolets National Medical University,
anesthesiology and intensive care department, Kyiv

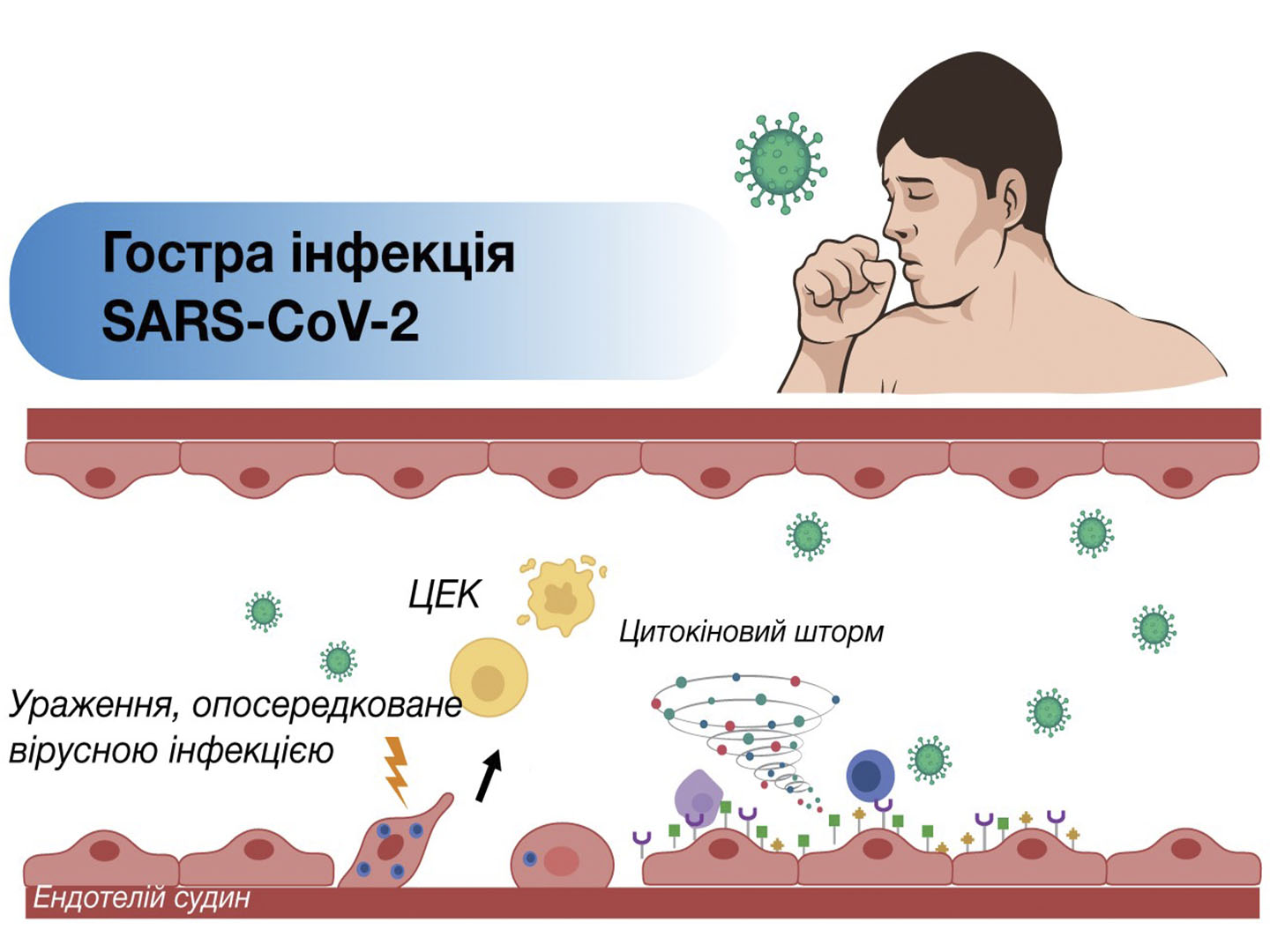
July 10, 1881 Landerer successfully performed injection of “sodium chloride physiological solution” to patient and ensured perpetuation for this infusion medium. Last century became an epoch of fluid management formation and development. This medical branch is successfully developing nowadays. In the 90-s years of last century crystalloids were generally made in hospital pharmacies, they had limited terms of storage. At present time there is a clear trend of finished pharmaceutical forms of infusion solutions production, they are produced in pharmaceutical factories. Fluid management is an important tool for complex treatment of many diseases.
Doctor must clearly know the aim of infusion solution use, the drug quantitative composition and have comprehensive information about its mechanism of action.
Maintaining normal blood plasma potassium concentration is one of the major conditions of a rational fluid management. Potassium is the main cation and osmotic component of intracellular fluid. Health adults have only 2% (60-80 mEq/L) of total body potassium (3000-4000 mEq/kg of body weight) in extracellular fluid [1]:
- • К+ cell concentration isabout 160 mmol/L, i. e. is 40 times higher than in extracellular fluid.
- • High intracellular К+ content ismaintained due to active transport (through Na/K-ATPase).
- • Potassium plays the definingrole in a cell bioelectrical activityand maintaining of neuromuscu-lar irritability and conductivity.
Potassium daily maintenance inhuman is 2.5-5 g or 0.7-0.9mmol/L/day. Total body potassium contain depends primarilyon muscle bulk, in female it islesser than in male; potassiumconcentration decrease is also observed in case of muscular atrophy. Potassium balance is de-fined by its intake and renal excretion. About 90% of entered into body potassium is excreted by kidneys, and the major portion is secreted by distal nephron. At the same time it is known, that potassium balance is based on the following mechanisms:
- • in abnormal conditions acidosiscontributes to intracellular potassium to hydrogen cations exchange (hyperkalemia), alkalosisfavours hypokalemia;
- • insulin and β-adrenergic effectscontribute to potassiumtransport into cells, α-adrenoceptor agonists – to potassium backflow;
- • mineralocorticoids or glucocorticoids excess intensify renal po-tassium excretion and influencepotassium distribution betweenintracellular and extracellularcompartments, accelerating itstransport into cells;
- • in the same cells of distal tu-bules simultaneously take placetwo oppositely directed pro-cesses: potassium reabsorptionand secretion, that provides max-imum potassium abstraction outof the urine in case of its blooddeficiency, and in case of excess –potassium excretion[2];
- • not only kidneys, but gastro-in-testinal tract and perspiratoryglands take part in potassium ex-cretion.
In case of human body potassiumdeficiency, there occur disturbances of normal kidney functionand histological changes gradually develop. Convoluted tubulesbecome more stretched, nephrones become vacuolated.There is noted a significant decrease of renal concentratingability, caused by the necessity ofpotassium ions restoring, andaciduria. Simultaneously, excessive sodium ion reabsorptiontakes place, this leads to its accumulation in the body. Homeo-static regulation of plasma potassium concentration is not so perfect, as one of, for example, sodium or glucose. Renal regulationmechanism efficiently preventshyperkalemia, but much worse works in conditions of hypokalemia. In clinical practice conditions accompanied by potassium deficiency are encountered more rarely, than hyperkalemia.