The first data on international multicenter clinical study RheoSTAT-CP0691 on the efficacy and safety of Rheosorbilact® infusion in therapy of purulent peritonitis

Abstract: Generalized forms of peritonitis are a major factor leading to non-traumatic mortality in all cases of emergency care and the second leading cause of sepsis in critically ill patients. The aim of this study was to evaluate the efficacy and safety of multicomponent infusion solution Reosorbilact in the treatment of patients diagnosed with purulent peritonitis.
An international multicenter randomized study included 181 patients aged from 18 to 60 years with a diagnosis of purulent peritonitis. Patients received therapy with Reosorbilact according to the prescribing information for use. The primary endpoint of the effectiveness was change in SOFA scale on day 3 therapy. Changes in APACHE II, SAPS II, MODS, and MPI scores as well as changes in endogenous intoxication markers on day 3 therapy were considered as secondary endpoints.
Safety was assessed by analysis of adverse events (AE) and vital signs after 3 days of therapy. On day 3 of treatment with Reosorbilact statistically significant changes were observed in SOFA (1.80±0.91 points), MODS (1.45±0.76 points) and MPI (1.84±5.03 points) scales. There was a statistically significant improvement in markers of endogenous intoxication (creatinine, bilirubin, white blood cell count, C-reactive protein, neutrophil to lymphocyte ratio) on day 3 of treatment. The majority of AE (98.99 %) were mild.
No AE were associated with the study preparation and did not result in the patient’s withdrawal from the study. According to the results of RheoSTAT-CP0691, Rheosorbilact is an effective and safe drug for the treatment of patients with purulent peritonitis. It is advisable to include Rheosorbilact in routine treatment algorithms for patients with purulent peritonitis.
Keywords: abdominal sepsis, septic shock, peritonitis, desintoxication therapy, infusion therapy, efficacy, safety, Reosorbilact.
Introduction
Acute generalized peritonitis is a life-threatening intra-abdominal pathology [1, 2]. Generalized forms of peritonitis are the major factor leading to non-traumatic mortality in all cases of emergency care and the second leading cause of sepsis in critically ill patients [6, 7]. In case of not providing the timely therapy, patients develop bacteremia, septicemia, septic shock and multiple organ dysfunction [4].
Therapy of purulent peritonitis, which may be accompanied by sepsis and multiple organ failure, is one of the most challenging and controversial issues in abdominal surgery. Over the last 10 years the incidence of purulent peritonitis has begun to increase, and expectations of solving the problem only with antibacterial agents were not realized. According to various studies, mortality from common forms of peritonitis ranges from 8 to 34% [1, 3, 4, 7, 8].
Despite significant advance in the efficacy of laboratory tests, imaging technologies, perioperative resuscitation and surgical techniques, the management of patients with common forms of peritonitis requires a comprehensive approach and becomes a challenges for surgeons and anesthesiologists [4, 5, 9].
Success in the treatment of peritonitis along with maintaining the leading role of early surgical intervention, timely elimination of the source of peritonitis, careful debridement and adequate abdominal drainage largely depends on suppressing infection with intensive antibiotic therapy, control of intoxication, elimination of intestinal paresis, prevention of secondary complications, and feasible pre- and postoperative intensive therapy aimed at eliminating hemodynamic disorders, correcting volumetric and metabolic changes, as well as restoring and maintaining the functions of vital organs and systems at the optimal level.
Current antibiotic therapy, methods of intra- and extracorporeal detoxification, undoubtedly, enable achieving certain positive results in the treatment of peritonitis, but the outcomes still cannot be considered satisfactory. Therefore, research and development of new therapeutic algorithms for detoxification and improvement of rheological blood parameters in patients with peritonitis are needed.
In line with the Infectious Diseases Association of America guidelines for the management of abdominal sepsis, patients with peritonitis should receive rapid fluid resuscitation to restore circulating blood volume (CBV). If septic shock develops, resuscitation should be started immediately after hypotension is detected. Patients without signs of decreased CBV should be treated by intravenous fluid therapy if intra-abdominal infection is suspected [10].
The consensus of the World Society for Emergency Surgery on the management of intra-abdominal infections states that the use of fluid therapy to improve microvascular blood flow and to increase blood volume per minute is an integral part of the abdominal sepsis treatment. The main goal of infusion therapy is to increase systemic blood pressure (BP). According to the consensus, the first-line drugs are economically accessible crystalloid infusion solutions characterized by a high tolerance profile [11].
After stabilization of hemodynamics (mean blood pressure ranging from 65 to 90 mm Hg) in patients with generalized peritonitis, it is recommended to use a restrictive type of fluid therapy, that is, to exclude the potential administration of an excessive volume of infusion, which can enhance abdominal edema, increase intra-abdominal pressure, as well as cause other adverse effects: from glycocalyx damage to reduced renal function [11–17].
Since it is extremely difficult to solve all the tasks of fluid therapy and to avoid undesirable fluid overload using mono-component solutions, the use of multicomponent infusion products is increasing. International multicenter clinical trial RheoSTAT was conducted to assess the efficacy of multicomponent fluid therapy in the complex treatment of peritonitis.
The object of the study was the multifunctional product Rheosorbilact® comprising: sorbitol 60 g, sodium lactate 19 g, sodium chloride 6 g, calcium chloride 0.1 g, potassium chloride 0.3 g, magnesium chloride 0.2 g, water for injection up to 1 Liter. The osmolarity is 891 mOsm/L, pH is 6.0–7.6. The objective of this study is to assess the following parameters in patients with purulent peritonitis, treated with Rheosorbilact:
- dynamics of points according to SOFA integral score (Sepsis-related Organ Failure Assessment), MODS (Multiple Organ Dysfunction) and MPI (Mannheim Peritonitis Index) scores;
- dynamics of biochemical parameters of endogenous intoxication;
- dynamics of immunological parameters of endogenous intoxication;
- dynamics of integral parameters of endogenous intoxication;
- safety of the investigational medicinal product in terms of incidence of adverse events (AEs) and overall survival.
Materials and methods
An electronic search in the PubMed, MEDLINE and Cochrane Library databases over the past 20 years was conducted using a sensitive strategy without language restrictions for the following keywords: “peritonitis”, “purulent peritonitis”, “septic shock”, “fluid resuscitation”, “sepsis resuscitation”, “infusion”. The results of the recently completed international multicenter open-label blinded end-point randomized controlled Phase III-IV ReoSTAT study (RCS) were also reviewed based on a report provided by Yuria-Pharm.
The RheoSTAT RCT included 629 patients with sepsis, peritonitis, burn disease, and pneumonia who were treated at 37 clinical centers in 6 countries. The ReoSTAT-CP0691 peritonitis sub-study included 181 patients from 10 clinical centers in 4 countries – Ukraine, Moldova, Georgia and Uzbekistan in accordance with the principles set out in the World Medical Association (WMA) Declaration of Helsinki, as well as the principles of Good Clinical Practice (ICH E6 GCP) and national standards of the member countries.
The procedure for obtaining patient’s informed consent was consistent with the national standards of the member countries, the requirements of Good Clinical Practice ICH E6 GCP and the ethical principles set out in the current version of the Declaration of Helsinki. The study design is shown in Figure 1.
Criteria for inclusion of patients to sub- study RheoSTAT-CP0691:
- men and women aged 18 and 60 years inclusively;
- 24 hours or less from the moment of established purulent peritonitis (diffuse or generalized, that is, in two or more anatomical parts of the abdominal cavity) by primary laparotomy and revision of the abdominal cavity with MPI index 21 to 29 points, which corresponds to the 2nd degree of severity and reactive phase according to unified clinical protocol for medical care for patients with acute peritonitis;
- informed consent for participation signed by the patient;
- baseline SOFA score of 2 points and higher.
The investigational medicinal product was Rheosorbilact®, solution for infusion, administered intravenously for 3 days at the doses (volumes) specified in the SmPC.
Data analysis was carried out in following populations:
- population of all included patients (intent-to-treat, ITT): all randomized patients who have been prescribed and administered at least one infusion of the investigational product and have data on SOFA scores both before and after administration of the investigational product or comparator;
- per protocol population (perprotocol, PP): all randomized patients who have completed the study accordingto the protocol (completed the proposed treatment and follow-up period without significant abnormalities);
- safety population: all randomized patients who have received at least one dose of the investigational product or comparator and completed at least one safety assessment visit.
Safety assessments were performed throughout the study. AE is defined as any medically adverse event experienced in study participant after administration of drug and may not be drug-related. That is, an AE could be any unfavorable symptom (including laboratory abnormalities), complaint or disease and which does not necessarily exclude a time relationship with this medicinal (investigational) product, regardless of the presence or absence of such relationship. A serious AE (SAE) considered as any medical undesirable event which:
- resulted in death;
- was a life threatening;
- led to persistent or substantialincapacity and disability;
- required hospitalization or prolongation of current hospitalization;
- caused a congenital anomaly/developmental defect.
In addition, any event that did not formally meet the above mentioned criteria but reported as significant medical event by investigator, was considered SAE. All other AEs that did not meet these criteria considered non-serious.
The study enrolled only 181 patients of which 90 were randomized to Rheosorbilact group. ITT population included 74/90 (82.22%) patients from Rheosorbilact group. Per-protocol population included 73/90 (81.11 %) patients. Safety population included all randomized patients.
The main efficacy endpoint in this study was a change in total SOFA score on Day 3 compared to baseline at admission, calculated as the difference in mean values (parameter value at enrollment minus parameter value at the end of treatment).
Additionally, a wide range of biochemical markers, immunological criteria and integral parameters of endogenous intoxication severity, characteristics of patients with advanced purulent peritonitis were assessed as secondary efficacy endpoints. The criteria for the efficacy and safety assessments are presented in Table 1.
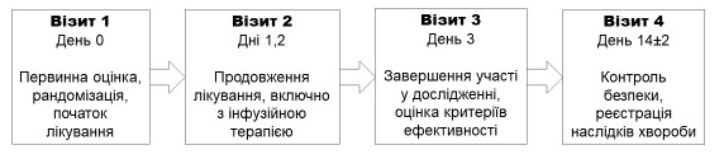 Figure 1. RheoSTAT-CP0691 Study Design Diagram
Figure 1. RheoSTAT-CP0691 Study Design Diagram
Visit 1 Day 0
Initial assessment, randomization, start of treatment
Visit 2 Days 1, 2
Continuation of treatment, including infusion therapy
Visit 3 Day 3
Completion of participation in the study, assessment of the efficacy criteria
Visit 4 Day 14±2
Safety control, record of the disease outcomes
Table 1. Efficacy and Safety Assessment Criteria in the RheoSTAT–CP0691 Study
|
The efficacy was assessed by comparing the baseline parameter values at admission and parameter values on Day 3 of therapy |
|
Primary endpoint: Change in the total SOFA score Secondary endpoints: • Changes in the total APACHE II, SAPS II, MODS and MPI scores • Evaluation of endogenous intoxication based on: – biochemical markers: serum concentrations of glucose, sodium, potassium, urea, creatinine, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase, alkaline phosphatase, lactate dehydrogenase, creatine phosphokinase, procalcitonin, albumin fraction, standard bicarbonate and lactate; – immunological criteria: assay of leukocytes, lymphocytes, platelets with the calculation of leukocytic, nuclear and hematological indices of intoxication (II), neutrophil to lymphocyte ratio, concentrations of C-reactive protein (CRP), immunoglobulins, interleukins 1 and 2, complement components 3 and 4; – clinical signs (adynamia, apathy, weakness, memory impairment, sleep disturbances, irritability, anorexia), ECG findings, central hemodynamics and assessment of consciousness on the Glasgow scale |
|
Safety assessment |
|
• Overall incidence rates of AEs • Incidence rates of SAEs • Incidence rates of drug-related AEs • Incidence rates of AEs leading to study withdrawal • Incidence rates of AEs previously listed in SmPC • Frequency of multiple organ failure • Overall survival (% ) during follow-up (Day 14 ± 2) |
Results and discussion
There are two main classes of infusion agents: colloids and crystalloids. Colloids include albumin, hydroxyethyl starch, and gelatin. Due to oncotic activity, colloids should theoretically slow down capillary leakage. However, in patients with severe infection this effect is rather short-term due to the glycocalyx damage [18, 19]. Compared to crystalloids, colloids have a slightly longer intravascular half-life, although capillary leakage affects both classes [20].
Other hypothetical benefits of colloids include anti-inflammatory effects and the ability to absorb nitric oxide, but this only applies to albumin [21]. To date, there is no large randomized, controlled trial (RCT) that demonstrates a clear difference in mortality between fluid therapy with crystalloids or colloids in pneumonia or sepsis. The SAFE RCT involving critically ill adults was large enough and compared 0.9% sodium chloride and albumin as means of fluid resuscitation.
Despite the lack of significant difference in 28-day mortality in the total group, the use of albumin led to the best results in patients with severe sepsis and acute respiratory distress syndrome, but the outcomes were worse in patients with severe traumatic brain damage [22, 23].
Hydroxyethyl starch solutions have been associated with acute kidney injury in critically ill individuals and are therefore considered hazardous in USA and Europe [24, 25]. Recent international guidelines for the management of sepsis do not recommend the use of colloids as an initial solution for fluid resuscitation due to the lack of benefits and excessive costs [26].
Unbuffered solutions (isotonic solution of sodium chloride) and multi-electrolyte buffered solutions can be distinguished among crystalloids. They differ in composition, chloride concentration, pH and osmolarity, but are more similar to plasma than isotonic solution of sodium chloride. Resuscitation with the use of 0.9% sodium chloride is associated with hyperchloremic metabolic acidosis, acute kidney damage and dangerous functional disorders of vital organs [27–30].
Despite this, isotonic sodium chloride remains the most common administered crystalloid solution [31], which is also often used as a solvent for various intravenous medicines [28]. Two recent RCTs, SALT-ED and SMART, suggest clear advantages of balanced buffers over isotonic sodium chloride solution. Although there was no difference in short-term mortality, administration of 0.9% sodium chloride was associated with a high risk of acute kidney damage, including death, need for dialysis, or long-term renal impairment [29, 30].
Particularly noteworthy are infusion solutions containing polyhydric alcohols, primarily sorbitol, which has a number of benefits:
- Due to slower conversion to monosaccharides, it is utilized better than glucose and does not cause carbohydrate overload;
- After administration, it is quickly involved into general metabolism (80% is utilized by the liver, 5% is deposited in the brain tissues, myocardium and skeletal muscle, the rest is excreted with urine or is used for urgent energy needs);
- It eliminates acetylcholine-induced intestinal spasm, stimulates intestinal motility without sharp increase, which justifies its use in the postoperative period;
- At a hypertonic concentration, it has a significant anti-edema effect, in particular, it promotes the reverse development of pulmonary edema, is characterized by an osmotic diuretic effect, which is important in the setting of oligoanuria and acute kidney damage;
- Due to its strong cholecystokinetic and choleretic effects, it restores the normal function of the digestive system, has a proven therapeutic effect in patients with acute and chronic hepatitis and toxic liver damage;
- At isotonic concentration it acts as antiaggregant, improving tissue microcirculation and perfusion.
A complex multipurpose infusion product Rheosorbilact® manufactured by Yuria-Pharm (Ukraine) is worth mentioning among sorbitol-containing agents. In addition to sorbitol, it contains other important electrolytes such as potassium, calcium and magnesium, but its chloride content is only 112.7 mmol/L, which reduces the risk of hyperchloremic acidosis. Another important ingredient of Rheosorbilact is sodium lactate, which provides an alkaline effect, increases the reserve and titratable blood alkalinity, corrects metabolic acidosis, which often complicates severe infections, sepsis, peritonitis, intestinal obstruction, renal failure, burns, shock, chronic hypoxia, etc.
It has positive effects on the cardiac function, regeneration and respiratory blood function, stimulates the mononuclear phagocyte system functions, has a detoxifying effect, enhances diuresis, improves the functioning of kidneys and liver. The concentration of sodium lactate in Rheosorbilact is 5–6 times higher (160–180 mmol/L) than in most infusion solutions, providing a strong therapeutic effect.
Two components of Rheosorbilact, having synergistic detoxification effects and the ability to correct acid-base and water-electrolyte balance, put this product on a par with the strongest detoxifying agents [32]. The successful experience with Rheosorbilact for detoxification and normalization of blood rheology in patients with such severe pyoinflammatory diseases as peritonitis [33], destructive pancreatitis [34], diabetic foot [35] suggests an improvement in the clinical consequences of pneumonia.
In addition, one of the clinical studies found that the administration of Rheosorbilact to patients with pneumonia promotes early normalization of body temperature, reversal of manifestations of as the no vegetative syndrome and reduction in the mean duration of hospitalization, stabilization of the acid-base state and coagulogram [36].
Change in the total SOFA score on Day 3 compared to baseline on admission
ITT population
On admission, the mean (± standard deviation) SOFA score was 2.38±0.59 points in Rheosorbilact group. On Day 3 the mean SOFA score was 0.58±0.84 points. The mean change in SOFA score on Day 3 from baseline was 1.80±0.91 points.
The additional analysis found that the changes in the mean SOFA score on Day 3 compared to the baseline were statistically significant (p <0.001) in patients receiving Rheosorbilact (Figure 1).
PP population
On admission, the mean (± standard deviation) SOFA score was 2.37±0.59 points. On Day 3 of treatment with Rheosorbilact, the mean SOFA score was 0.55±0.80 points. The mean change in SOFA score on Day 3 compared to the baseline was 1.82±0.89 points (Figure 2).
Change in the total MODS score on Day 3 compared to baseline on admission
ITT population
On admission, the mean (± standard deviation) MODS score was 2.22±1.11 points. On Day 3 the mean MODS score was 0.77±1.27 points in Rheosorbilact group. The mean change in the MODS score on Day 3 compared to the baseline was 1.45±0.76 points.
The additional analysis found that the changes in the mean MODS score on Day 3 compared to the baseline were statistically significant (p <0.001) in patients in Rheosorbilact group (Figure 1).
PP population
On admission, the mean (± standard deviation) MODS score was 2.21±1,12 points in Rheosorbilact group. On Day 3, the mean MODS score was 0.75±1.27 points. The mean change in the MODS score on Day 3 compared to the baseline was 1.45±0.76 points (Figure 2).
Change in the total MPI score on Day 3 compared to baseline at admission
ITT population
On admission, the mean (± standard deviation) MPI score was 2.61±2.27 points. On Day 3 the mean MPI score in patients receiving Rheosorbilact was 20.77±5.43 points. The mean change in the MPI score on Day 3 compared to the baseline was 1.84±5.03 points.
Additional analysis found that the changes in the mean MPI score on Day 3 compared to the baseline were statistically significant (Figure 2).
PP population
On admission, the mean (± standard deviation) MPI score was 22.62±2.28 points. On Day 3, the mean MPI score in patients receiving Rheosorbilact was 20.92±5.31 points. The mean change in the MPI score on Day 3 from baseline was 1.70±4.92 points (Figure 3).
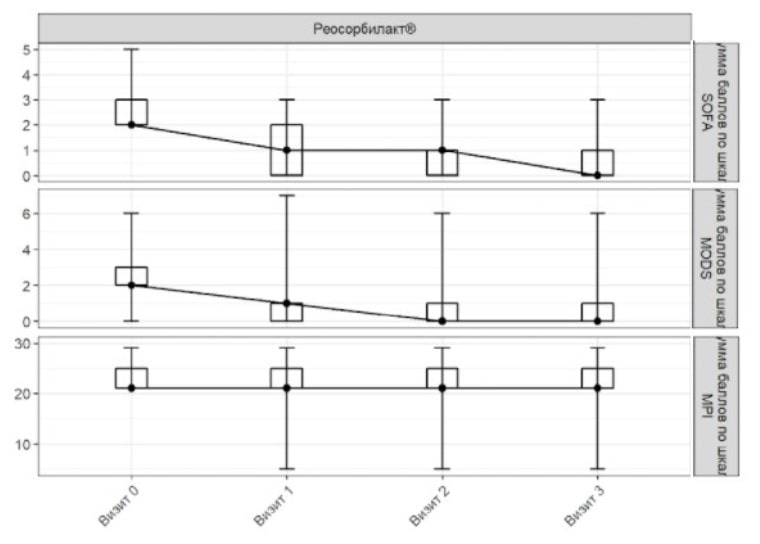 Figure 2. SOFA, MODS and MPI scores in ITT population
Figure 2. SOFA, MODS and MPI scores in ITT population
Visit 0 Visit 1 Visit 2 Visit 3
Total SOFA score Total MODS score
Total MPI score Rheosorbilact
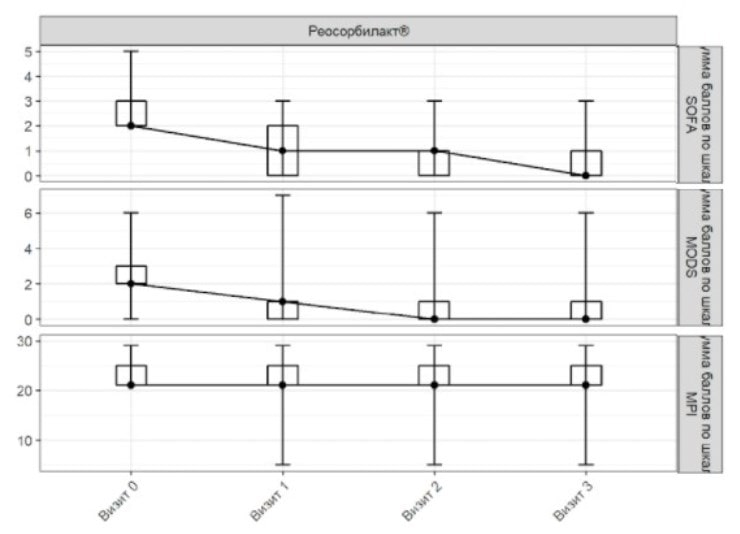 Figure 3. SOFA, MODS and MPI scores in PP-population
Figure 3. SOFA, MODS and MPI scores in PP-population
Visit 0 Visit 1 Visit 2 Visit 3
Total SOFA score Total MODS score
Total MPI score Rheosorbilact
Changes in markers of endogenous intoxication in ITT population (WBC, lymphocytes, CRP, bilirubin and creatinine)
On admission, the median (interquartile range) WBC was 10.95 (9.62–11.95) × 109/L in Rheosorbilact group. On Day 3, the median (interquartile range) WBC was 7.10 (6.00–8.43) × 109/L in Rheosorbilact group. The additional analysis found that the changes in WBC count on Day 3 compared to the baseline were statistically significant (p <0.001).
On admission, the median (interquartile range) CRP concentration in Rheosorbilact group was 10.00 (6.00–14.00) mg/mL. On Day 3, the median (interquartile range) CRP concentration in the Rheosorbilact group was 0.00 (0.00–21.00) mg/mL. The additional analysis found that the changes in the mean CRP concentration on Day 3 compared to the baseline were statistically significant (p <0.001).
On admission, the median (interquartile range) neutrophil to lymphocyte ratio was 4.44 (4.00–7.61) in Rheosorbilact group. On Day 3, the median (interquartile range) neutrophil to lymphocyte ratio was 4.08 (3.64–4.59) in Rheosorbilact group. The additional analysis found that the changes in the mean neutrophil to lymphocyte ratio on Day 3 compared to the baseline were statistically significant in the groups (р <0.001).
On admission, the median (interquartile range) total bilirubin concentration was 16.00 (12.00–17.90) μmol/L in Rheosorbilact group. On Day 3, the median (interquartile range) total bilirubin concentration was 11.15 (7.00–15.82) μmol/L in Rheosorbilact group. The additional analysis found that the changes in the mean total bilirubin concentration on Day 3 compared to the baseline were statistically significant (p <0.001).
On admission, the median (interquartile range) creatinine concentration was 102.00 (84.50–109.00) mmol/L in Rheosorbilact group. On Day 3, the median (interquartile range) creatinine concentration was 90.00 (70.00–102.00) mmol/L in Rheosorbilact group. The additional analysis found that the changes in the mean creatinine concentration on Day 3 compared to the baseline were statistically significant (active treatment group: p <0.001).
The changes in the blood biochemistry in the PP population on Day 3 of therapy is shown in Figure 4.
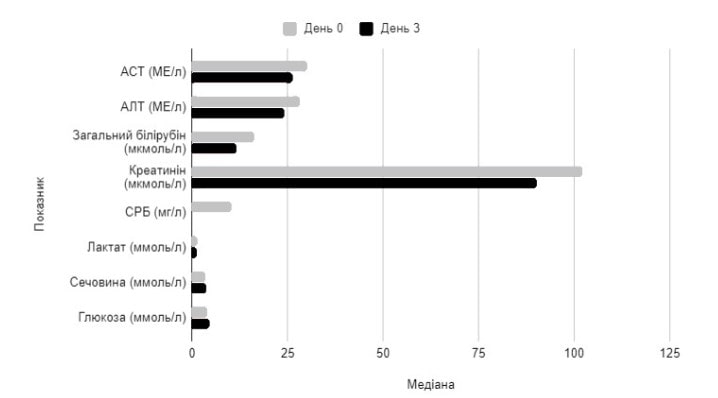 Figure 4. Changes in median values of blood chemistry parameters on Day 3 of Rheosorbilacttherapy in PP population
Figure 4. Changes in median values of blood chemistry parameters on Day 3 of Rheosorbilacttherapy in PP population
Parameter Median
Day 0 Day 3
AST, IU/L ALT, IU/L
Total bilirubin, μmol/L
Creatinine, μmol/L
CRP, mg/L Lactate, mmol/L
Urea, mmol/L Glucose, mmol/L
Analysis of Adverse Events
During the study, AEs were reported in 63 patients (890 AEs) of Rheosorbilact group. A total of 1 AE was reported and considered to be related to the investigational medicinal product in 1 patient in the active treatment group. A total of 3 SAEs were reported (2 SAEs in Rheosorbilact group). No AEs were considered as drug-related and did not result in withdrawal of patient from the study. Based on safety analysis results, Rheosorbilact has an acceptable safety profile.
Of 890 AEs observed in Rheosorbilact group, 881 AEs (98.99%) were mild, 6 AEs (0.67%) were moderate, and 3 were severe AEs (0.34%). No SAEs leading to patient’s study withdrawal were observed. No drug-related SAEs were reported; SAEs most likely were related to underlying disease (purulent peritonitis). On Day 14 of the study, the overall survival in Rheosorbilact group was 100%.
Vital signs
On admission, the median (interquartile range) body temperature was 37.00°C. On Day 3 of the therapy, the median (interquartile range) body temperature was 36.60 (36.60–36.80) °C.
On admission, the median (interquartile range) heart rate (HR) was 88.00 (82.50–90.00) bpm. On Day 3 of the therapy with Rheosorbilact, the median (interquartile range) HR was 82.00 (76.00–86.00) bpm.
On admission, the median (interquartile range) systolic blood pressure (SBP) was 115.00 (110.00–120.00) mm Hg. On Day 3 of the therapy, the median (interquartile range) SBP was 120.00 (115.00–120.00) mm Hg.
On admission, the median (interquartile range) diastolic blood pressure (DBP) was 75.00 (70.00–80.00) mm Hg. On Day 3 of the therapy with Rheosorbilact, the median (interquartile range) SBP was 75.00 (70.00–80.00) mm Hg.
On admission, the median (interquartile range) respiratory rate (RR) was 18.00 (17.00–18.00)/min. On Day 3 of the therapy, the median (interquartile range) RR was 18.00/min (17.00–18.00).
Electrocardiography findings
On admission, the mean (± standard deviation) HR was 88.00±11.40 bpm. On Day 3 of the therapy, the mean (± standard deviation) HR was 82.12±11.67 bpm.
On admission, the median (interquartile range) PR interval was 106.00 (102.00–141.50) ms. On Day 3, the median (interquartile range) PR interval was 126.00 (104.00–146.00) ms.
On admission, the median (interquartile range) QRS width was 0.10 (0.09–0.10) s. On Day 3, the median (interquartile range) QRS width was 0.10 (0.09–0.10) s.
On admission, the median (interquartile range) QT interval was 0.35 (0.33–0.37) s. On Day 3, the median (interquartile range) QT interval was 0.36 (0.34–0.38) s.
On admission, the median (interquartile range) QTc interval was 0.39 (0.37–0.42) s. On Day 3, the median (interquartile range) QTc interval was 0.40 (0.38–0.42) s.
On admission, 5.68% (n=5/90) of patients had clinically significant abnormal ECG findings. On Day 3, 2.27% (n=2/90) of patients had clinically significant abnormalities.
Already on Day 3 of therapy, most patients showed normalization of body temperature, renal function and an improvement in most assessment scores used in the study (Table 2).
Table 2. Efficacy parameters of Rheosorbilact before and after therapy*
|
Parameters, units |
Baseline |
On Day 3 |
P |
||||
|
n |
Me |
IQR |
n |
Me |
IQR |
||
|
Total score |
|||||||
|
SOFA |
74 |
2 |
2–3 |
74 |
0 |
0–1 |
<0.001 |
|
APACHE II |
74 |
3 |
2–5 |
74 |
3 |
1–4 |
0.118 |
|
SAPS II |
74 |
14 |
8–18 |
74 |
14 |
8–16 |
0.121 |
|
MODS |
74 |
2 |
2–3 |
74 |
0 |
0–1 |
<0.001 |
|
MPI |
74 |
21 |
21–25 |
74 |
21 |
21–25 |
0.005 |
|
Body temperature, °С |
74 |
37 |
36.80–37.40 |
74 |
36.6 |
36.6–36.8 |
<0.001 |
|
HR, bpm |
74 |
88.5 |
84.25–90.00 |
74 |
82 |
78.25–88.00 |
<0.001 |
|
SBP, mm Hg |
74 |
115 |
110–120 |
74 |
115 |
110–125 |
0.331 |
|
DBP, mm Hg |
74 |
75 |
70–80 |
74 |
75 |
70–80 |
0.071 |
|
RR per 1 min |
74 |
18 |
17–18 |
74 |
17 |
17–18 |
0.006 |
|
Urea, mmol/L |
74 |
3.4 |
3.00–4.50 |
74 |
3.60 |
3.00–4.58 |
0.477 |
|
Creatinine, μmol/L |
74 |
102.00 |
84.50–109.00 |
74 |
90.00 |
80.00–102.00 |
<0.001 |
|
Total bilirubin, μmol/L |
74 |
16.00 |
12.00–17.90 |
74 |
11.15 |
7.00–15.82 |
<0.001 |
|
ALT, IU/L |
74 |
28.00 |
22.00–30.00 |
74 |
24.00 |
20.00–30.00 |
0.022 |
|
AST, IU/L |
74 |
30.00 |
24.00–32.75 |
74 |
26.00 |
22.00–32.00 |
0.015 |
|
Albumin fraction, % |
15 |
63.80 |
58.00–65.25 |
15 |
61.50 |
57.22–63.87 |
0.397 |
|
CRP, mg/L |
73 |
10.00 |
6.00–14.00 |
73 |
0.00 |
0.00–10.00 |
<0.001 |
|
Platelets, ×109/L |
74 |
210.00 |
190.00-235.25 |
74 |
220.00 |
194.00–301.25 |
<0.001 |
|
WBC, ×109/L |
74 |
10.95 |
9.62–11.95 |
74 |
7.10 |
6.00–8.43 |
<0.001 |
|
Nuclear II |
62 |
0.05 |
0.05–0.09 |
63 |
0.05 |
0.03–0.06 |
<0.001 |
|
Leukocytic II |
62 |
4.20 |
2.37–4.31 |
63 |
1.64 |
0.79–4.10 |
<0.001 |
|
Hematological ІІ |
62 |
4.00 |
3.82–4.32 |
63 |
3.35 |
2.57–4.00 |
<0.001 |
|
Neutrophil/lymphocyte ratio |
62 |
4.44 |
4.00–7.61 |
64 |
4.08 |
3.64–4.59 |
<0.001 |
Notes:* data from the report on results of RCT RheoSTAT-CP0691, provided by Yuria-Pharm; n – number of observations; Me: (IQR) – median (interquartile range).
Discussion
Considering the fact that patients with degree 2 purulent peritonitis are assessed as having sepsis, as primary endpoint for the efficacy therapy was selected SOFA score, which allows quantifying severity of organ-system disorders and monitoring efficacy of therapy in patients with sepsis over time. Since the population of patients with purulent peritonitis and sepsis is heterogeneous not only in the nature and severity of organ failure, but also in age and comorbidities, which is reflected in the course of the disease, the MODS scores were used as secondary efficacy endpoints. MPI index was used to assess the severity of peritonitis over time.
As a result of the assessment of the primary efficacy endpoint, a change in the total SOFA score on Day 3 compared to the baseline (on admission), in the population of all enrolled patients (basic population for assessing the primary efficacy endpoint), the mean change in SOFA score on Day 3 compared to the baseline was 1.80±0.91 points. Similar results were obtained in the per protocol population: the mean change in SOFA score on the 3rd day compared to the baseline was 1.82±0.89 points.
The additional analysis of the parameter changes on Day 3 compared to the baseline found that in the Rheosorbilact group there was a statistically significant decrease in the severity of multiple organ failure and the severity of the condition, according to the scores such as SOFA, MODS II and MPI, a decrease in the severity of endogenous intoxication, assessed by biochemical (creatinine and bilirubin concentrations) and immunological (WBC, CRP, neutrophil to lymphocyte ratio) markers, as well as a decrease in the mean values of the body temperature and heart rate.
In the Rheosorbilact group, statistically significant changes were also observed on Day 3 compared to the baseline parameters of endogenous intoxication as AST, ALT, medium molecular weight oligopeptides, platelet count, and a decrease in RR.
Notably, already on Day 3 of therapy, the subjects of the active treatment group showed a decrease in the percentage of deviations of some laboratory indicators of the function of elimination organs (Figure 5).
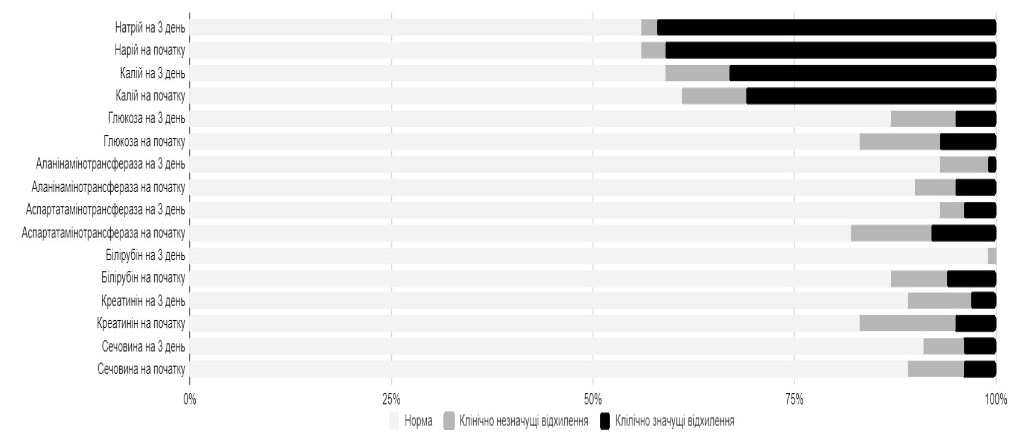 Figure 5. Percentage of abnormalities inelimination organs, blood glucose and blood electrolyte levels before and after 3-days treatment with Rheosorbilact
Figure 5. Percentage of abnormalities inelimination organs, blood glucose and blood electrolyte levels before and after 3-days treatment with Rheosorbilact
Reference Clinically insignificant abnormalities Clinically significant abnormalities
Sodium on Day 3 Baseline sodium
Potassium on Day 3 Baseline potassium
Glucose on Day 3 Baseline glucose
ALT on Day 3 Baseline ALT
AST on Day 3 Baseline AST
Bilirubin on Day 3 Baseline bilirubin
Creatinine on Day 3 Baseline creatinine
Urea on Day 3 Baseline urea
Thus, the obtained results suggest that Rheosorbilact effectively improves the condition of patients, reduces the severity of multiorgan failure and endogenous intoxication, according to most of the parameters evaluated in the study in patients with purulent peritonitis.
Conclusion
The current evidence base and recent international guidelines favor balanced crystalloid infusion solutions as a pathogenetic therapy for generalized peritonitis and abdominal sepsis. According to the results of the open blinded end-point ReoSTAT RCS, administration of Rheosorbilact® to patients with purulent peritonitis pneumonia (intravenous infusion at a dose of 200-400 ml/day for 3 days) effectively improves the clinical condition, reduces the manifestations of (multi-) organ failure and endogenous intoxication.
The efficacy of Rheosorbilact was expressed in a statistically significant improvement of integral parameters of the patient outcomes based on SOFA, MODS and MPI scores. Low-volume infusion therapy with Rheosorbilact (200–400 ml per day) increased CBV and reduced the total volume of infusion required to achieve a therapeutic effect, with no risk of hyperhydration and fluid overload, which is especially important in critically ill patients.
Lactate component of Rheosorbilact (exogenous) does not affect the level of endogenous lactate, which determines favorable safety profile of the solution. As part of intensive care, administration of Rheosorbilact contributed to decrease the hyperthermia, heart rate and WBC count, the signs of endogenous intoxication. Administration Rheosorbilact during first 3 days of intensive therapy provided fast and safe normalization of rheological blood parameters in patients with purulent peritonitis. Consequently, Rheosorbilact is an effective and safe treatment in patients with purulent peritonitis.
The RheoSTAT-CP0691 study substantiates the feasibility of using Rheosorbilact in the complex therapy of purulent peritonitis. It is advisable to include Rheosorbilact in routine treatment algorithms for patients with purulent peritonitis.
Authors:
S. Agop1, V. Sharipova2, K. Kashibadze3, D. Vashadze4, V. Tevdoradze5, I. Kolosovich6, S. Peev7, A. Ligonenko8, V. Cojocaru9, A. Bely10
Literature:
- AdesunkanmiA.R.K., OseniS.A., Adejuyigbe O., et al. Acute generalized peritonitis in African children: assessment of severity of illness using modified APACHE II score. ANZ J. Surg. 2003; 73: 275-9.
- Drexel S., Tseng D. Primary peritonitis: an index case of mycoplasma hominis infection in a healthy female. Case Rep. Surg. 2018; 2018: 1-4.
- Ghosh P., Mukherjee R., Sarkar S., et al. Epidemiology of secondary peritonitis: analysis of 545 cases. Int. J. Sci. Stud. 2016; 3: 83-8.
- Ross J.T., Matthay M.A., Harris H.W. Secondary peritonitis: principles of diagnosis and intervention. BMJ. 2018; 361: k1407.
- Strobel O., Werner J., Büchler M. Surgical therapy of peritonitis. Chirurg. 2011; 82: 242-8.
- Sartelli M., Catena F., Ansaloni L., et al. Complicated intra-abdominal infections worldwide: the definitive data of the CIAOW study. World J. Emerg. Surg. 2014; 9: 37.
- Chichom-Mefire A., Fon T., Ngowe-Ngowe M. Which cause of diffuse peritonitis is the deadliest in the tropics? A retrospective analysis of 305 cases from the south-west region of Cameroon. World J. Emerg. Surg. 2016; 11: 14.
- Adesunkanmi A.R.K., Badmus T.A. Pattern of antibiotic therapy and clinical outcome in acute generalized peritonitis in semi-urban and rural Nigerians. Chemotherapy. 2006; 52: 69-72.
- Søreide K., Thorsen K., Søreide J. Strategies to improve the outcome of emergency surgery for perforated peptic ulcer. Br. J. Surg. 2014; 101: e51-64.
- Armstrong C. Updated guideline on diagnosis and treatment of intra-abdominal infections. American Family Physician. 2010 Sep 15; 82 (6).
- Sartelli M., Catena F., Moore E. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World Journal of Emergency Surgery. 2017; 12: 22.
- Hippensteel J.A., Uchimido R., Tyler P.D., Burke R.C., Han X., Zhang F., et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit. Care. 2019; 23 (259). https://doi.org/10.1186/s13054–019–2534–2.
- Cordemans C., De Laet I., Van Regenmortel N., Schoonheydt K., Dits H., Huber W., et al. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann. Intensive Care. 2012; 2: S1.
- Malbrain M.L., Marik P.E., Witters I., Cordemans C., Kirkpatrick A.W., Roberts D.J., et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 2014; 46: 361-80.
- Heung M., Wolfgram D.F., Kommareddi M., Hu Y., Song P.X., Ojo A.O. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol. Dial. Transplant. 2012; 27: 956-61.
- Bouchard J., Soroko S.B., Chertow G.M., Himmelfarb J., Ikizler T.A., Paganini E.P., et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009; 76: 422-7.
- Stein A., de Souza L.V., Belettini C.R., Menegazzo W.R., Viégas J.R., Costa Pereira E.M., et al. Fluid overload and changes in serum creatinine after cardiac surgery: predictors of mortality and longer intensive care stay. A prospective cohort study. Crit. Care. 2012; 16: R99.
- Best M.W., Jabaley C.S. Fluid management in septic shock: a review of physiology, goal-directed therapy, fluid dose, and selection. Curr. Anesthesiol. Rep. 2019; 9: 151-157. https://doi.org/10.1007/s40140-019-00330-3.
- Marik P., Bellomo R. A rational approach to fluid therapy in sepsis. Br. J. Anaesth. 2016; 116 (3): 339.
- Hahn R., Lyons G. The half-life of infusion fluids: an educational review. Eur. J. Anaesthesiol. 2016; 33 (7): 475-82.
- Caironi P., Tognoni G., Masson S., Fumagalli R., Pesenti A., Romero M., et al. Albumin replacement in patients with severe sepsis or septic shock. N. Engl. J. Med. 2014; 370 (15): 1412-21.
- Finfer S., Bellomo R., Boyce N., French J., Myburgh J., Norton R., et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004; 350 (22): 2247-56.
- SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J., Cooper D., Finfer S., Bellomo R., Norton R., Bishop N., Kai Lo S., Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N. Engl. J. Med. 2007 Aug 30; 357 (9): 874-84. doi: 10.1056/NEJMoa067514.
- Myburgh J.A., Finfer S., Bellomo R., Billot L., Cass A., Gattas D., et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N. Engl. J. Med. 2012; 367 (20): 1901-11.
- Perner A., Haase N., Guttormsen A., Tenhunen J., Klemenzson G., Åneman A., et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N. Engl. J. Med. 2012; 367 (2): 124-34.
- Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 2017; 45 (3): 486-552.
- MacDonald N., Pearse R. Are we close to the ideal intravenous fluid? Br. J. Anaesth. 2017; 119 (suppl. 1): i63-71.
- Magee C.A., Bastin M.L.T., Laine M.E., Bissell B.D., Howington G.T., Moran P.R., et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit. Care Med. 2018; 46 (8): 1217-23.
- Self W.H., Semler M.W., Wanderer J.P., Wang L., Byrne D.W., Collins S.P., et al. Balanced crystalloids versus saline in noncritically ill adults. N. Engl. J. Med. 2018; 378 (9): 819-28.
- Semler M.W., Self W.H., Wanderer J.P., Ehrenfeld J.M., Wang L., Byrne D.W., et al. Balanced crystalloids versus saline in critically ill adults. N. Engl. J. Med. 2018; 378 (9): 829-39.
- Dalton C. Why did sterile salt water become the IV fluid of choice? NPR. 2018. Available at: https://www.npr.org/sections/health-shots/2018/03/31/597666140/why-did-sterile-salt-water-become-the-iv-fluid-of-choice. Accessed: 02/04/2018.
- Кондрацкий Б., Новак В. Опыт применения в клинической практике комплексного инфузионного препарата Реосорбилакта. Искусство лечения. 2006; № 1: с. 34–36.
- Хамидов Д.Б., Косимов З.К., Хомидов Д.Д., Киямов С.Э. Комплексный полифункциональный раствор Реосорбилакт в интенсивной терапии эндогенной интоксикации у больных острым перитонитом. Научно-практический журнал ТИППМК. 2011; № 2: с. 77–79.
- Алиев Н.А., Бобиев А.Б., Хамидов Д.Б., Баротов Э.Д., Буриев Т.Н., Курбанов Д.А. и др. Реосорбилакт и Латрен в коррекции эндогенной интоксикации и оксидантного стресса у больных острым деструктивным панкреатитом. Медицина неотложных состояний. 2015; том 64, № 1: с. 57–59.
- Проничев В., Стяжкина С., Михайлов А. Эффективность лечения Реосорбилактом пациентов с синдромом диабетической стопы. Здоровье, демография, экология финно-угорских народов. 2016; № 2: с. 30–32.
- Рижко О.О. Инфузионная терапия Реосорбилактом. Укр. пульмонологический журнал. 2002; № 1: с. 94–96.