Final Evaluation of the Results of the СТІКс Study (Concomitant Xavron Stroke Therapy)
Abstract. Ischemic stroke remains a pressing problem today. Its pathogenesis consists of a sequential cascade of reactions in the brain, which, in addition to ischemia, are responsible for further damage to brain tissue and slow down the development of compensatory and regenerative mechanisms.
Attempts to break the pathological cascade have been going on for decades. The first promising molecule that demonstrated the potential of a scavenger (cleaner, absorber) of excessive aggressive peroxides in preclinical studies was MCI-186, which is used in clinical practice under the name edaravone.
The aim of the study the results of which are presented in this paper was to establish the clinical effects of edaravone (Xavron) as a concomitant therapy of acute ischemic stroke (СТІКс) in real clinical practice.
Background
Effects on the damaging mechanisms of active oxygen radicals in the hyperacute period of stroke (including cases of recanalization therapy) may increase the potential for recovery in the future.
Aim
Assess the impact of radical scavenger use on the long-term effects of stroke in routine clinical practice.
Material and methods
The open case-control study covered 53 stroke departments in Ukraine. 570 patients with ischemic stroke received additional therapy with “Xavron” (edaravone, Yuria-Pharm, Ukraine) – 30 mg twice daily, by infusion, during the hospitalization staying, starting 1 hour after admission (active treatment group, ATG).
430 patients corresponding by age and sex received conventional treatment (control group, CG). The average length of hospital stay was 9.690.19 days. The average score on NIHSS in the ATG was 11.670.26 and 10.930.29 in CG. The number of thrombolysis procedures was 25 (4.4%) in ATG and 30 (6.98%) in CG.
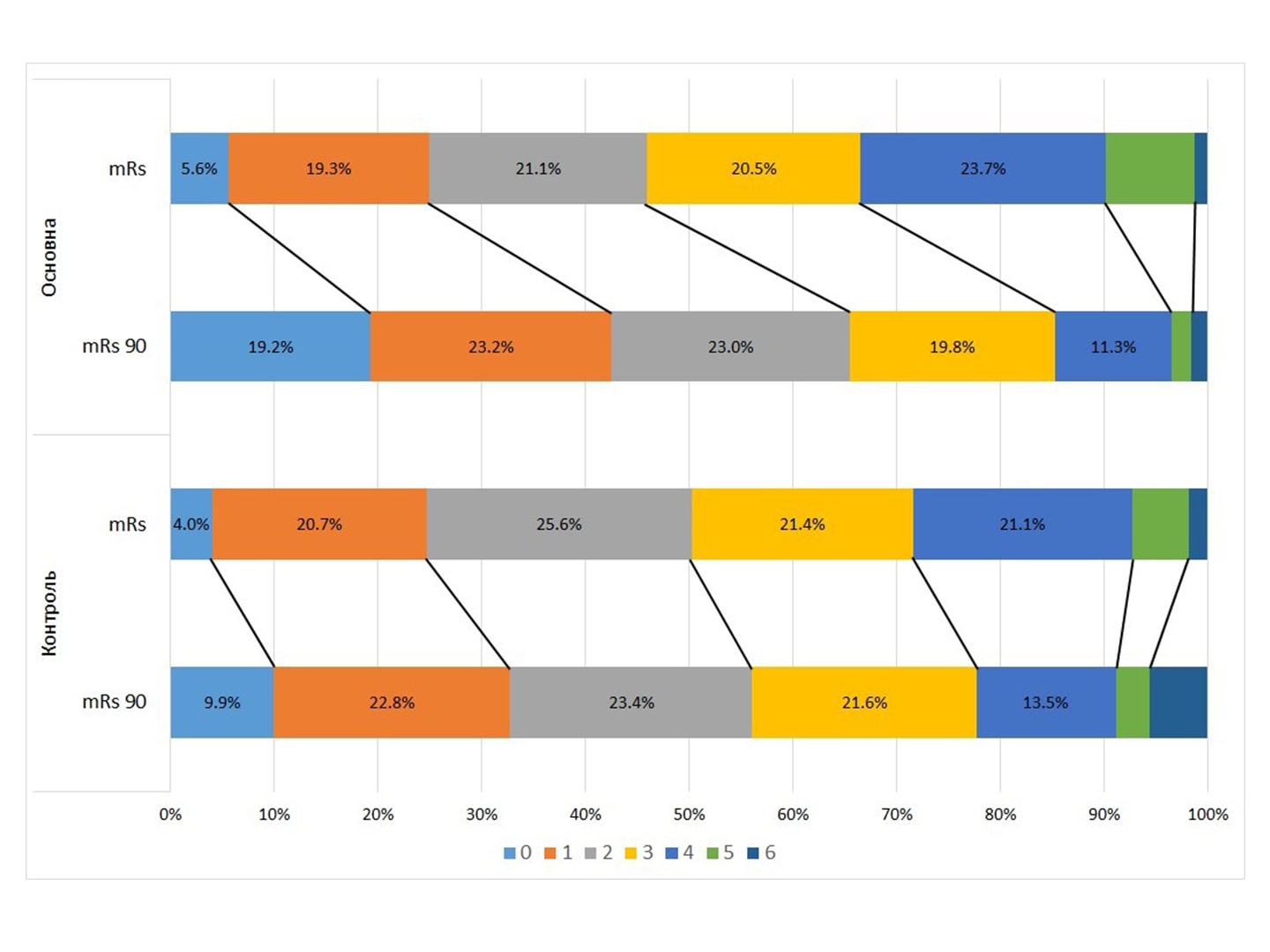
Results. At the time of discharge, the NIHSS score was 6.340,22 in ATG versus 7.460.27 in CG, p=0,001. The mean mRS score was almost the same at discharge (2.660.06 in ATG and 2.770.22 in CG). But after 3 months, the average score of mRS in ATG was significantly lower – 1.871.36 against 2.561.49, p <0.01. Dichotomous comparison of mRS 0-1 vs. 2-6: 42.47% in ATG vs. 32.74% in CG, p = 0.0018. For mRS 0-2 / 3-6: 65.4% in ATG and 56.1% in CG, p = 0.0029.
Positive dynamics of mRS “-1 point” for 90 days was observed in 62.6% of ATG patients against 34.8% in CG: OR = 2.42 (95% CI – 2.42 – 4.17). Hemorrhagic transformation of stroke was observed in 9 (1.84%) ATG patients and 22 (5.67%) CG patients, p = 0.0012. Hospital mortality was 0.7% in ATG and 2% in CG, p = 0.0713.
Conclusions
Additional therapy with free radical scavengers is perspective approach to strengthening the restorative potential after stroke.
Keywords: ischemic stroke, oxygen radicals, treatment, edaravone.
Authors:
S.P. Moskovko, O.V. Kyrychenko, H.S. Rudenko et al. National Pirogov Memorial Medical University, Vinnytsya, Ukraine
Literature:
- Московко С.П., Кириченко О.В., Руденко Г.С. Протокол відкритого багатоцентрового дослідження «випадок — контроль» щодо безпеки та ефективності використання едаравону (Ксаврон®) у гострому періоді ішемічного інсульту в умовах реальної клінічної практики. Здоров’я України. Спецвипуск «Інсульт». 2020. 1(52). С. 15-16.
- Ganesh A., Luengo-Fernandes R., Wharton R.M., Rothwell P.M. Ordinal vs dichotomous analysis of modified Rankin Scale, 5-year outcome, and cost of stroke. Neurology. 2018. 91. e1951-e1960. Doi: 10.1212/WNL.00000000006554.
- Chen C., Li M., Lin L., Chen S., Chen Y., Hong L. Clinical effects and safety of edaravone in treatment of acute ischaemic stroke: A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2021 Feb 27. doi: 10.1111/jcpt.13392.
- Московко С.П., Кириченко О.В., Руденко Г.С. Проміжні результати дослідження СТІКс (Супутня Терапія Інсульту Ксавроном) — відкритого багатоцентрового дослідження «випадок — контроль». Журнал неврології ім. Б.М. Маньковського. 2021. Т. 9. № 1.
- Watanabe K. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? Journal of Clinical Biochemistry and Nutrition. 2018. Vol. 62. Issue 1. P. 20-8. https://doi.org/10.3164/ jcbn.17-622.





