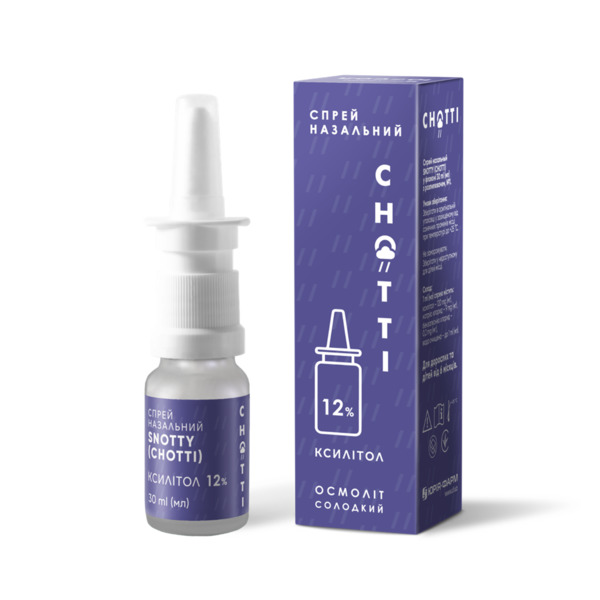
Snotty nebules
SNOTTY xylitol irrigation is a modern step in elimination-irrigation therapy!
Therapeutic indications:
- As an adjunct to the therapy for infectious and allergic conditions of the nasal cavity, paranasal sinuses, and nasopharynx.
- As a product that promotes the removal of nasal secretions and the elimination of nasal congestion in the case of difficult nasal breathing.
- For daily nasal hygiene.
SNOTTY is intranasal xylitol for irrigation of nasal passages and sinuses in upper respiratory tract infections and allergies.
Xylitol irrigation is a modern step in elimination-irrigation therapy. The use of xylitol irrigation is reasonable and substantiated based on the properties and effects of intranasal xylitol.
Important effects of SNOTTY xylitol irrigation in acute infectious or allergic nasopharyngitis:
- Quickly eliminates congestion and helps reduce the need for decongestants.
- Prevents the development of complications, otitis in particular.
- Helps reduce the disease duration.
- It is expedient starting from the first manifestations of a runny nose, as often and for as long as it is necessary.
Also, SNOTTY is sweet!
SNOTTY in containers is approved for use by children from birth! Babies’ noses need special care. A gentle droplet of SNOTTY gently and safely reaches even the smallest nostrils.
Release form:
- Solution in polymer containers of 2 ml, № 10.
How supplied
Sales markets
Ukraine
Instruction
INSTRUCTIONS FOR USE
of medical device
SNOTTY® Nasal Solution
Composition
1 mL of solution contains:
- Xylitol – 120 mg/mL
- Sodium chloride – 9 mg/mL
- Water for injection – up to 1 mL
Nature and contents of container
Solution in 2 mL polymer containers, 10 x 2 mL containers.
Instructions for use of medical device.
Description
SNOTTY® is a solution that cleans the nasal passages and sinuses, eliminating pollutants and irritants. SNOTTY®®) quickly relieves nasal congestion in patients with upper respiratory tract infections and allergies.
Xylitol is the main component of the medical device. In conditions of low pH, xylitol stabilizes the protein structures of cells. Due to its osmotic properties, it regulates salt metabolism on the mucosal surface of the nose and nasopharynx. High xylitol concentrations (12 %) prevent microorganisms from adhering to the mucosal cells and forming biofilms and have an antimicrobial effect on some pathogens.
Xylitol liquefies and facilitates the removal of mucus and retains moisture, whereby moisturizing and soothing irritated nasal mucosa, facilitating breathing and improving the quality of sleep in patients with acute and chronic infectious and allergic conditions of the nasal cavity, paranasal sinuses, and nasopharynx. The presence of xylitol provides a sweet taste.
The concentrations of the medical device components ensure the solution’s hyperosmolarity, removing excess moisture and eliminating mucosal swelling of the nasal passages and sinuses.
The components of the medical device do not react with the human body and are inert; they do not exert pharmacological, immunobiological, or metabolic effects and do not cause addiction.
No preservative has been added.
Sterile.
Intended use
SNOTTY®® is a solution for intranasal administration that reduces mucosal swelling in the nasal passages and sinuses and facilitates cleansing, thinning, and improving breathing through the elimination of secretions.
Target population
Children, including infants, and adults.
Therapeutic indications
As an adjunct to the therapy for infectious and allergic conditions of the nasal cavity, paranasal sinuses, and nasopharynx.
As a product that promotes the removal of nasal secretions and the elimination of nasal congestion in the case of difficult nasal breathing.
For daily nasal hygiene.
Contraindications
Hypersensitivity to any component of the medical device.
Undesirable effects
Hypersensitivity, burning sensation of the nasal mucosa.
In case of any adverse reaction, the medical device should be discontinued immediately, and the physician and manufacturer should be informed.
Special warnings and precautions for use
- Use SNOTTY® as per the instructions for medical use. Do not use in any other way than that described in the instructions for use of medical device. In case of any questions, consult your physician prior to use.
- Do not use the same polymer container for multiple patients. The use of one polymer container by more than one individual can cause the spread of infection.
- The product is intended for single use only. Repeated use may lead to infection. Do not re-use.
- Re-sterilization is not allowed.
- Do not use concomitantly with other products for intranasal administration. If there is a need for intranasal use of another medical device or medicinal product, there should be an interval of at least 15 minutes between administrations.
- Do not heat the solution container. When stored in the refrigerator, if necessary, the device should be kept for 15 to 30 minutes at ambient temperature prior to use.
- Do not mix with any solutions, medicines, etc.
- There are no available clinical data on the efficacy of the medical device and tolerance to it during pregnancy and breastfeeding. This patient population should consult a physician prior to the use of the medical device.
- Prior to use, check the integrity of the package and the shelf life. Do not use the device if the original packaging is damaged or if the shelf life has expired.
- Dispose in accordance with the local waste disposal requirements.
Interactions
Not detected.
Overdose
No cases of overdose have been reported.
Method of administration
Use 2 to 4 times daily. If needed, the dosage frequency can be increased. The duration of use should be estimated individually.
Children shall self-administer the product under the supervision of adults.
Method of administration for adults:
- prior to procedure, wash your hands with soap and carefully free the nasal passages from secretions by quickly exhaling sharply through the nose, while blocking one nasal passage with a finger;
- open the polymer container, and instill 2 to 4 drops of the solution into each nasal passage (breathe through the mouth or hold the breath while inhaling); when instilling into the right nasal passage, the head should be thrown slightly back and tilted to the left, and vice versa, the head should be tilted to the right when instilling into the left nasal passage;
- after instillation, it is recommended to stay in a supine position with a head thrown back for 2 minutes, then free the nasal passages from the liquefied secretions.
Method of administration for children:
- prior to procedure, wash your hands with soap and carefully free the nasal passages of the child from secretions;
- open the polymer container, and instill 2 to 4 drops of the solution into each nasal passage (ask the child to breathe through the mouth or hold the breath while inhaling); when instilling into the right nasal passage, the head of the child should be thrown slightly back and tilted to the left, and vice versa, the head of the child should be tilted to the right when instilling into the left nasal passage;
- after instilling, it is advisable for the child to stay in a supine position with a head thrown back for 2 minutes, then seat the child to keep his/her head straight and help to free the nasal passages from the liquefied secretions.
Special precautions for storage
Store in the original container at 25°С or below, protect from light. Do not freeze. Keep out of reach of children.
Shelf life
3 years. Shelf life is valid when the storage conditions in an original container are maintained.
Name and address of the manufacturer
Yuria-Pharm LLC, 10, M. Amosova Street Kyiv, 03038, Ukraine
Tel: +38 (044) 275 92 42, +38 (044) 275 01 08.
E-mail: uf@uf.ua
www.uf.ua
Manufacturing site: 108, Kobzarska Street, Cherkasy, 18030, Ukraine
Tel.: +38 (044) 281-01-01.
In case of any complaints on a medical device or feedback, please use one of the following ways:
- send a message to feedback@uf.ua;
- send a text message to +38 (095) 275 33 01 using the Viber, Telegram or WhatsApp applications;
- call +38 (095) 275 33 01 (rates according to your operator’s charges) or +38 (0800) 401 771 (free of charge from any number in Ukraine).
Issue date: 22.01.2024.
Version: 01.