This area is designed to create platforms for the production of syringes pre-filled with biopolymer gels. Principles of aseptic processing of raw pharmaceutical materials serve as the basis for the development of production technologies in compliance with GMP and ISO standards which allows to achieve product high quality and its safety for the patient.

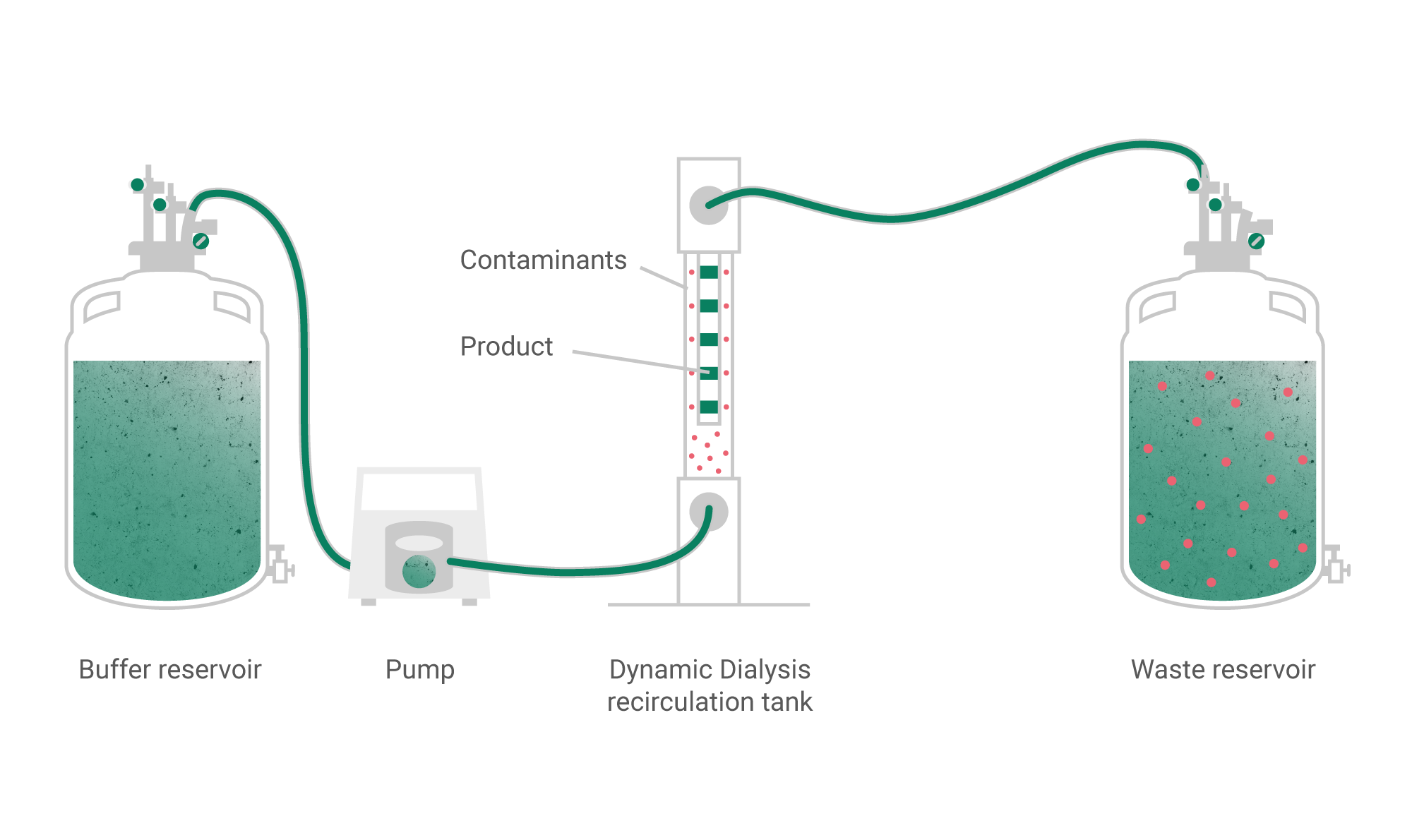
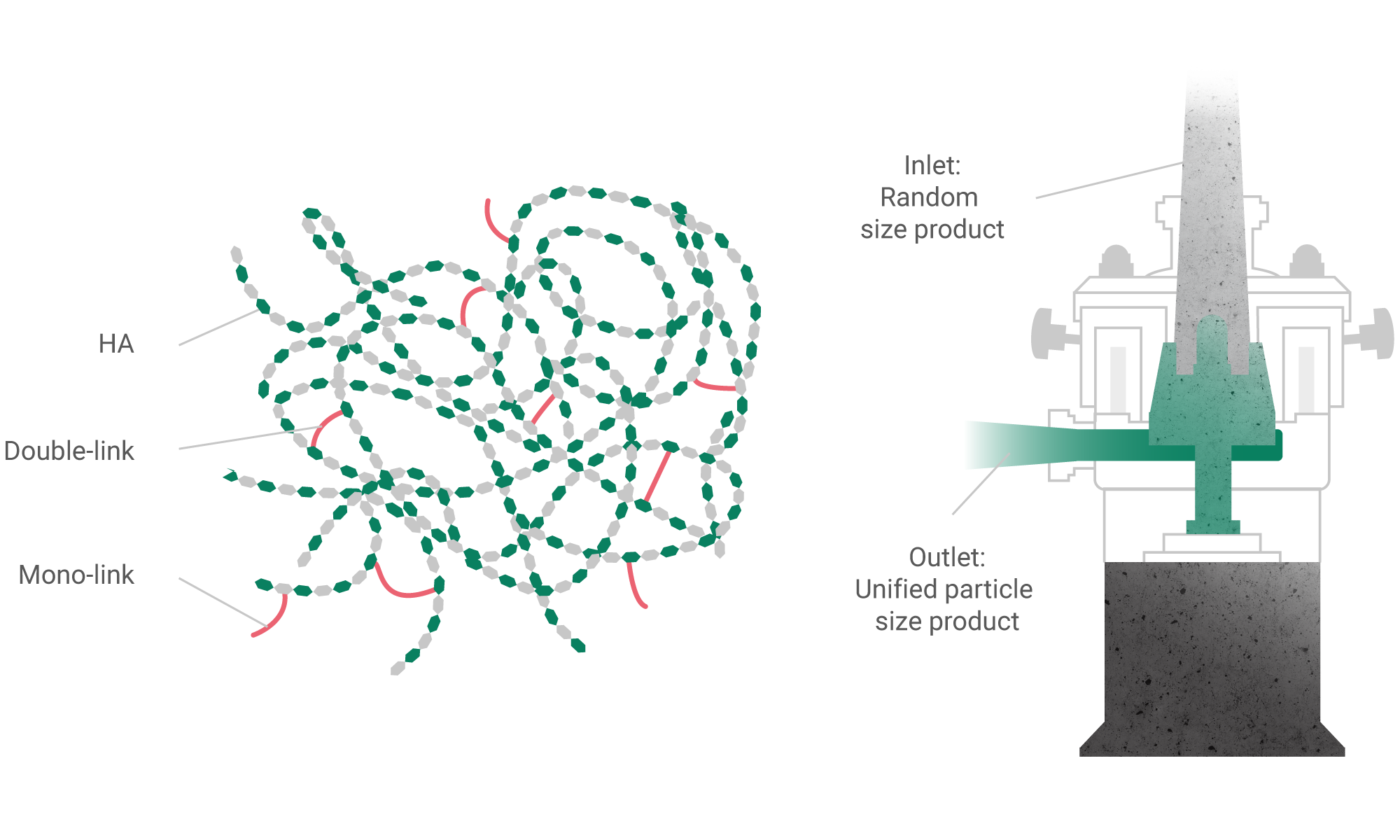
Classical methods of chemical synthesis in combination with the dialysis technology for purification from low molecular weight toxic agents allow to produce safe and effective solutions for injectable implants based on hyaluronic acid and other biopolymers.