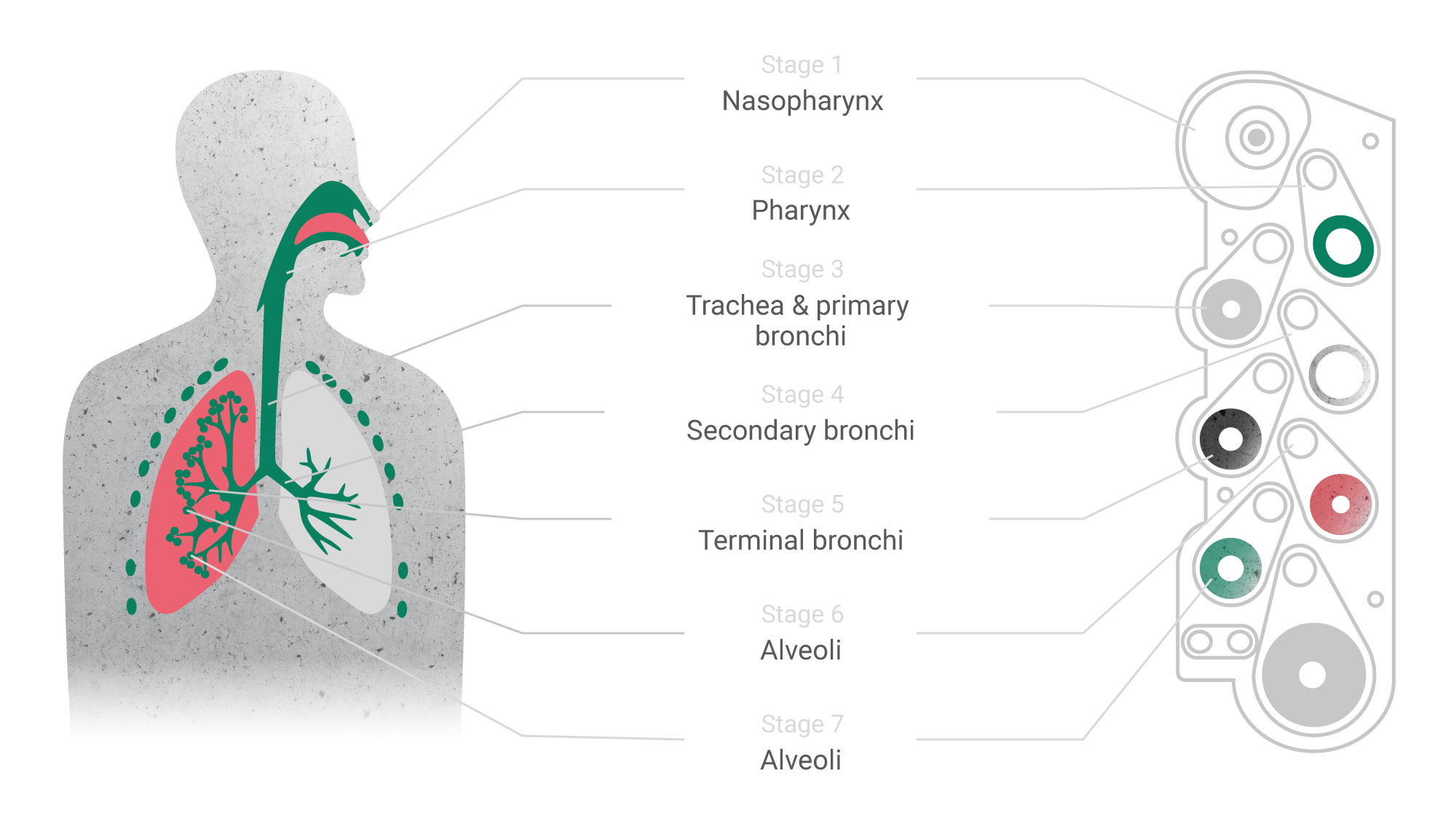
The rise of modern technologies has given a new impetus to the development of such traditional dosage forms as inhalation. On one hand, inhaled drugs may be the optimal approach for targeted delivery of active substances in treatment of respiratory diseases. On the other hand, the respiratory administration route is promising for drugs of systemic action: thanks to the extremely developed system of capillaries and easily permeable epithelial layer, absorption of drugs in the respiratory tract occurs much faster than in the gastrointestinal tract. Moreover, blood from the lungs immediately enters the left atrium through the small circle of blood circulation and is then evenly distributed throughout the body.
The nasal route of administration is promising for drug delivery to the brain, as it allows to bypass the blood-brain barrier through the so-called “nose-brainbarrier”.
The YURіA-PHARM R&D team is developing inhalation products in the following areas:
Inhalation solutions for nebulizer therapy
Inhalation suspensions for nebulizer therapy
Drug delivery devices (inhalators of various types)
Inhalation solutions for nebulizer therapy
The main idea of these products is the delivery of drugs (antibiotics, corticosteroids, adrenoblockers, mucolytics, etc.) directly into the respiratory tract. We are developing a comprehensive solution that includes an administration device (nebulizer) and a wide range of inhalation products. The interactions between inhalation products in different combinations and with the nebulizer itself are carefully studied to ensure full compatibility, efficiency and safety of nebulizer inhalations.
Inhalation suspensions for nebulizer therapy
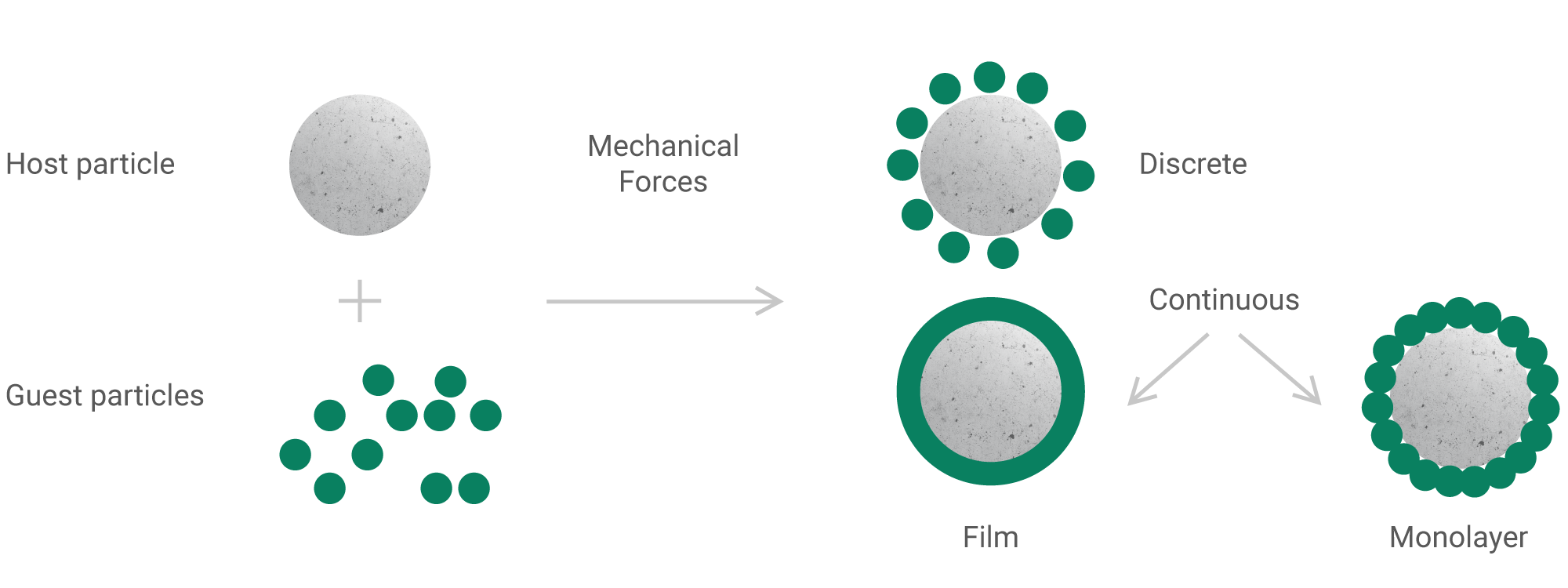
Unlike inhalation solutions, suspensions require proof of equivalence in vitro and/or in vivo. To achieve complete therapeutic equivalence with the original drug, production technological parameters of are carefully selected at the development stage and are thoroughly controlled during production. In particular, equivalence may be affected by the particle size distribution of the active substance, its polymorphic form, the size of droplets formed in the process of nebulization and other parameters.
Dry powder inhalations
This is one of the new types of inhalation products, the advantage of which is the ability to deliver the active substance to a specific part of the respiratory tract, including the smallest bronchioles and alveoli. This is achieved by selecting optimal particle size and structure of the active substance and the carrier which, in turn, affects the inertia and speed of movement in the airways. However, such effects are possible only when a narrow set of parameters (parameters design space) is reached and maintained at all production stages. The transfer stage is critical for such complex products, as it has to prove the reproducibility and validity of the technological process.
Nasal sprays
A traditional dosage form that includes products for spraying into the nasal cavity. A promising area of research is the development of a line of sprays that can deliver drugs directly to the brain through a narrow area located in the olfactory nerve. The development of such products provides possibilities for the treatment of nervous system and anesthesia, although requiring serious laboratory, preclinical and clinical studies. Currently, the YURіA-PHARM R&D team is at the laboratory stage of such research.

Inhalation anesthesia drugs
A highly specialized group of products, in the development of which we focus on proving their compatibility with the packaging and means of administration. We also pay considerable attention to the quality control methods, that may often include non-standard techniques, as compared to other products.
Drug delivery devices (inhalators of various types)
Along with other exprets, YURіA-PHARM has a team of engineers and programmers responsible for creating mechanical and electronic devices for the delivery of various types of inhalants.
Inhalation products are developed in specialized facilities equipped with mixers and homogenizers of different types and devices for product filling that are used for the technological process establishment. To develop methods of quality control, in addition to the traditional pharmaceutical chromatographic and spectrophotometric equipment, we use laser diffractometers, powder rheometers and equipment to determine zeta potential. Equivalence studies with the original products are carried out in a new-generation cascading impactor generation and in respiratory simulators.